|
The myriad of chemical
reactions occurring in the cell are collectively called
metabolism. Metabolism can be divided into two categories:
1) catabolism, the breakdown of molecules, and 2)
anabolism, the synthesis of new molecules. We will look at
two aspects of metabolism: 1) enzymes, and 2) energy and
metabolism. (We will cover some aspects of the last
section of chapter 3, biosynthesis, later.)
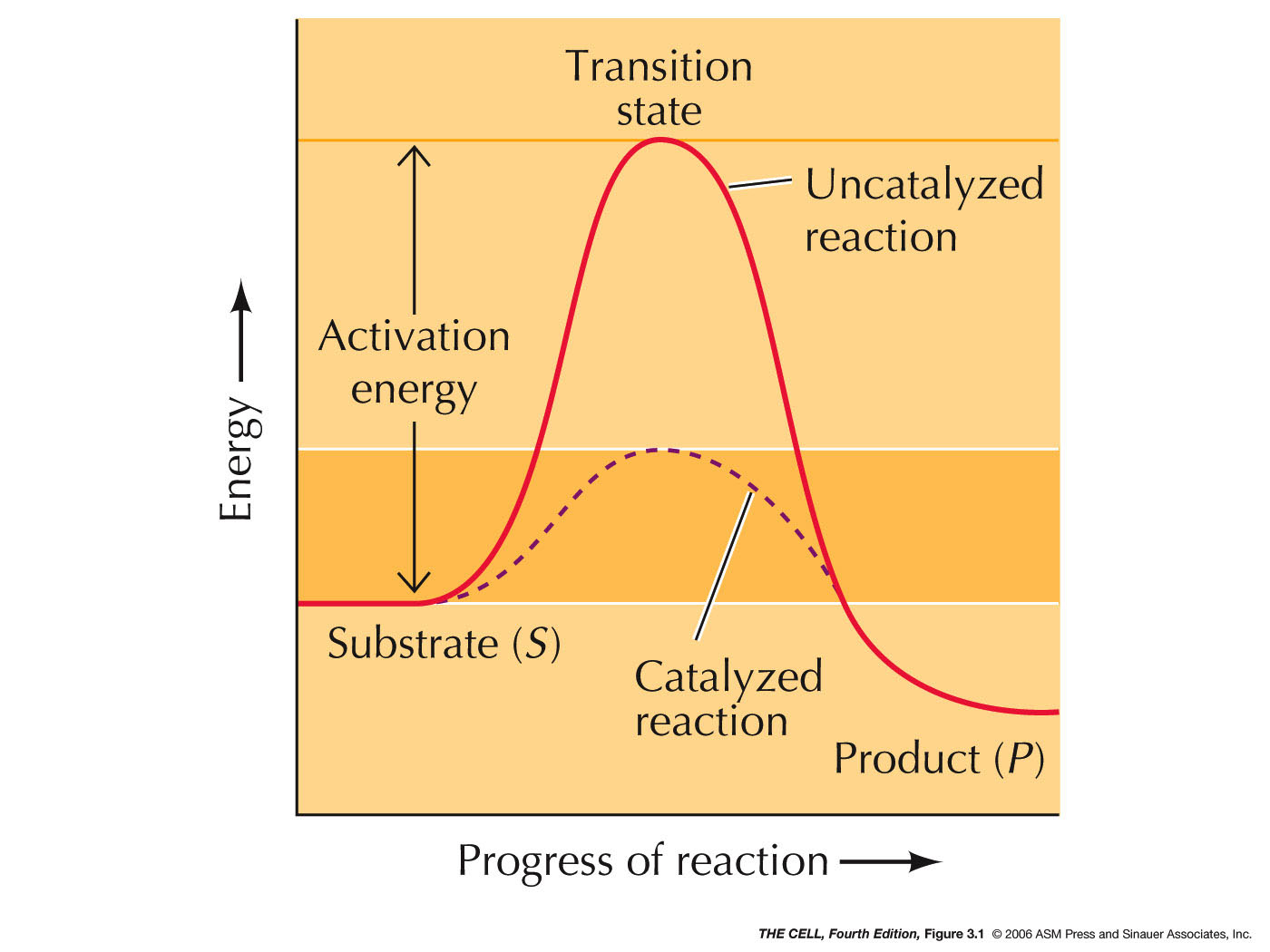
- Enzymes: Enzymes
are protein catalysts. (Note: there are also catalytic
RNAs.) A catalyst increases the rate of a chemical
reaction without itself being permanently changed.
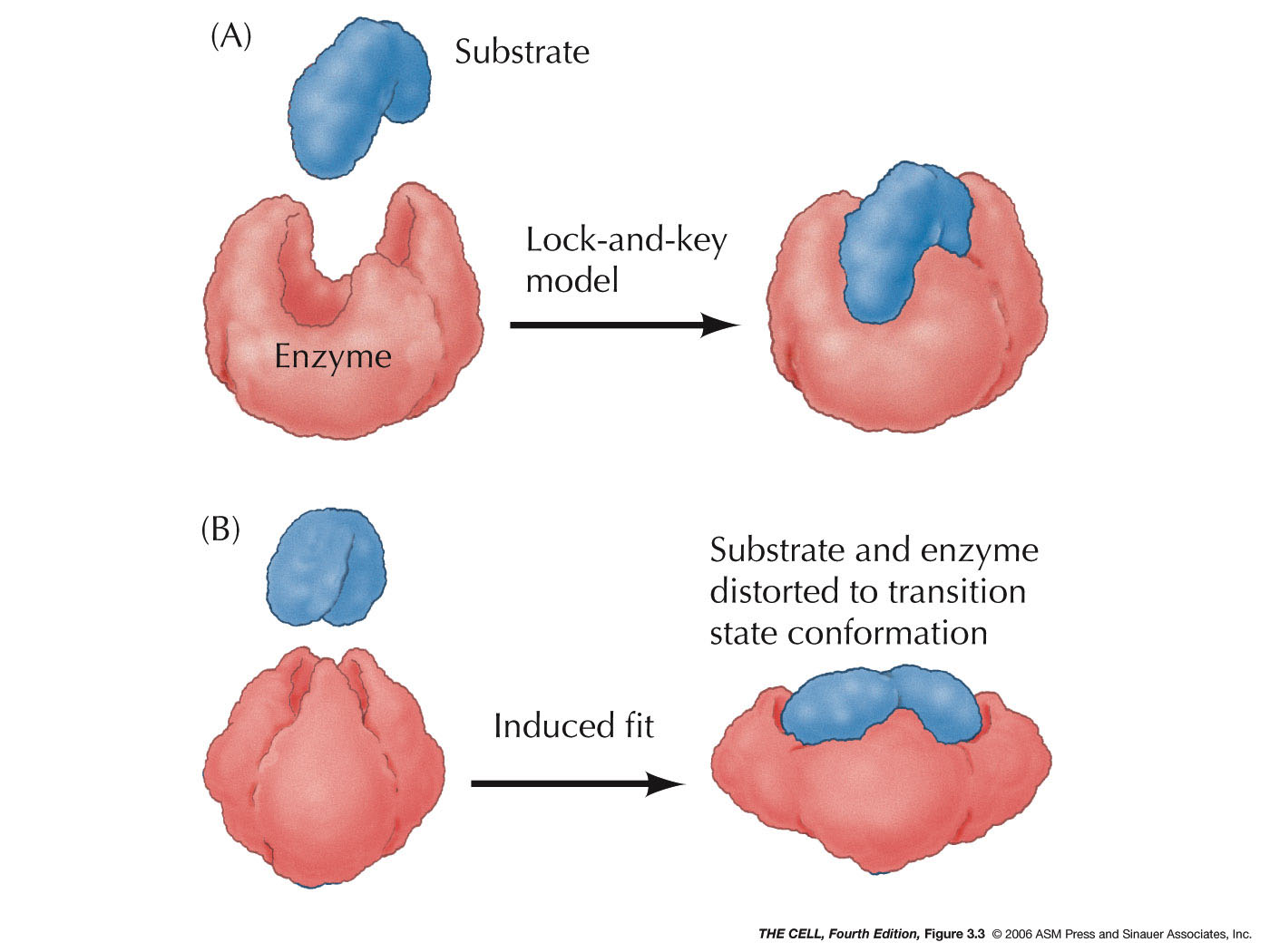
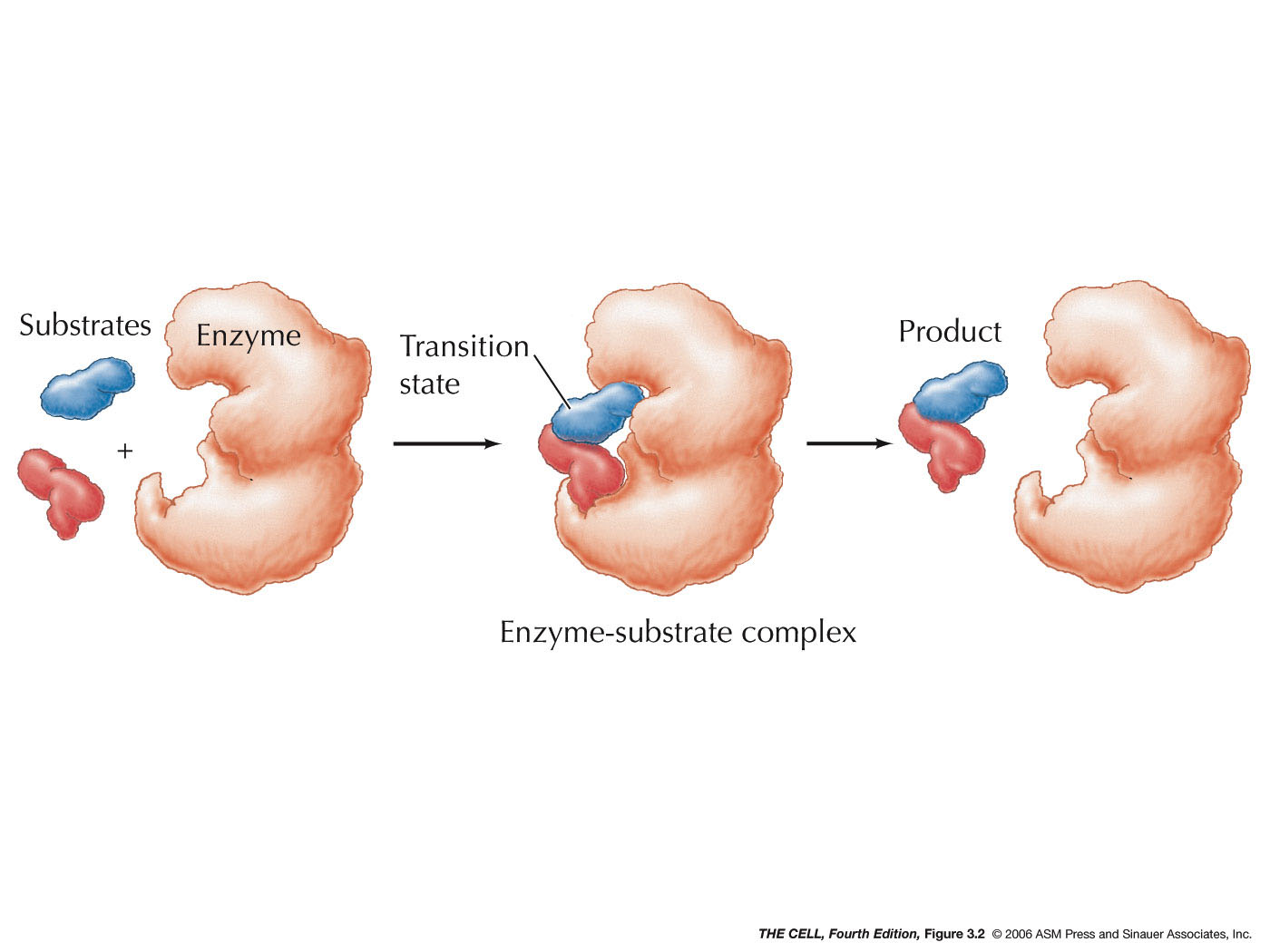
Enzymes bind (lock and key
binding) to a substrate
(or substrates). After the reaction, an end product (or
end products) is (are) released. While the enzyme may
have been altered in the intermediate substrate-bound
stage, it is released unaltered and can be reused.
Therefore, a small amount of enzyme can catalyze
numerous reactions.
|
Enzymes are things invented by biologists that
explain things which otherwise require harder
thinking. --
Jerome Lettvin
|
|
|

 |
|
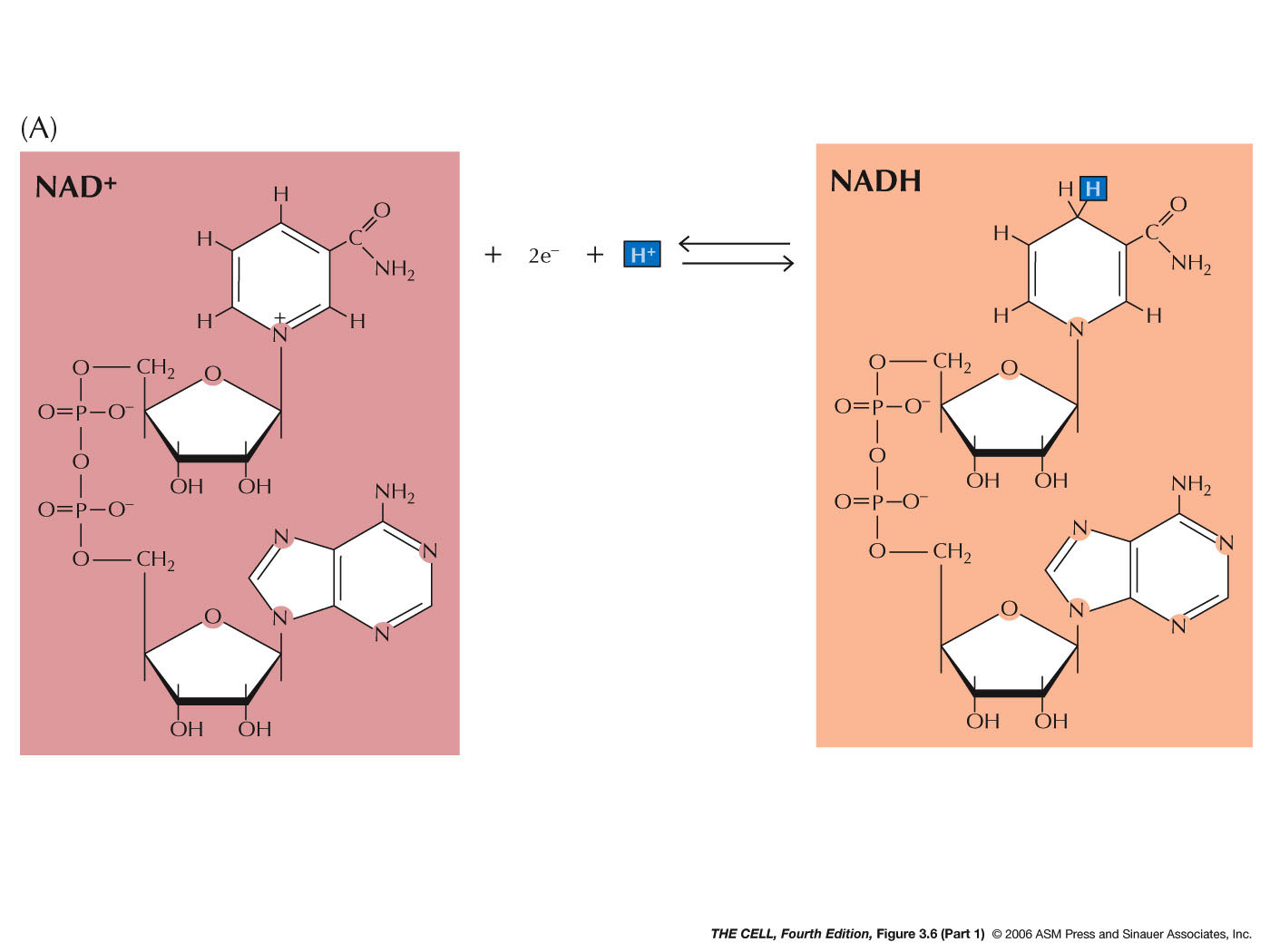
- Coenzymes:
A coenzyme is a type of prosthetic group (small,
non-protein molecules bound to proteins, like heme).
Coenzymes are small organic molecules that further
increase reaction rates. One example is the nucleotide
called nicotinamide adenine dinucleotide (NAD+)
which can be reduced (to NADH) by receiving one H+ and
two electrons and then oxidized (back to NAD+)
by donating these to another molecule. The
reduction/oxidation of NAD+/NADH is coupled to the
oxidation/reduction of other molecules. NAD+
---> NADH requires energy. NADH ---> NAD+
releases energy. (Video)
Similarly, the nucleotide called flavine adenine
dinucleotide (FAD) can be reduced to FADH2
(requiring energy) and FADH2
can be oxidized to FAD (releasing energy).
|
 |
|
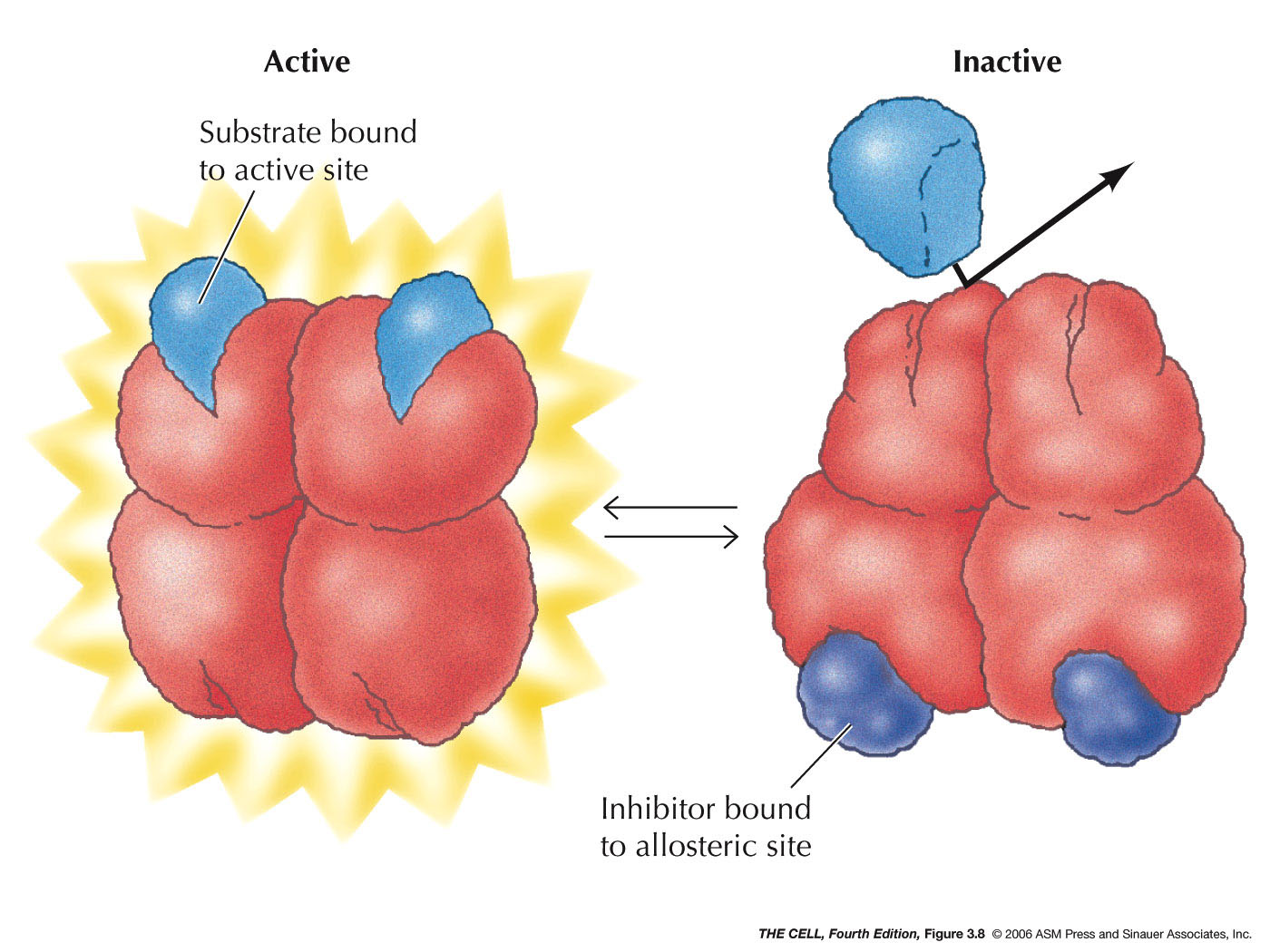
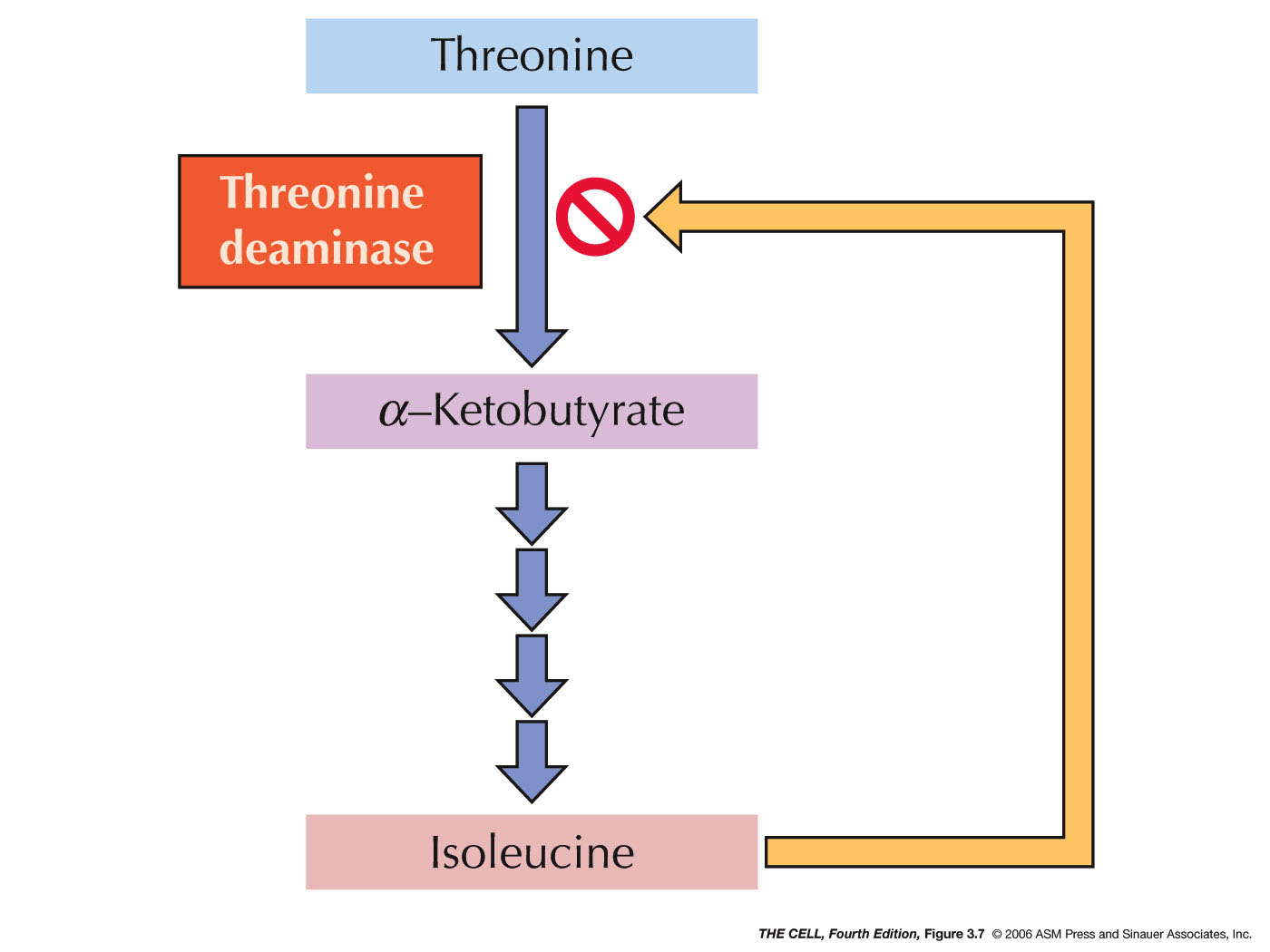
- Enzyme
Inhibition/Activation: Enzymes are often
inactivated by the presence of a pathway end product
that binds to a site other than the catalytic site (allosteric binding). This
is feedback inhibition.
(F.E.
Video.) Enzymes may also be activated or
deactivated by the addition of phosphate groups to a
serine, theonine, or tyrosine amino acid side chain.
This is phosphorylation.
The activity of enzymes responsible for
phosphorylation (a protein kinase) may in turn be
regulated by phosphorylation (another protein kinase).
(Video
at 5:50) Enzymes that remove phosphates are
called protein phosphatases.
|
 |
|
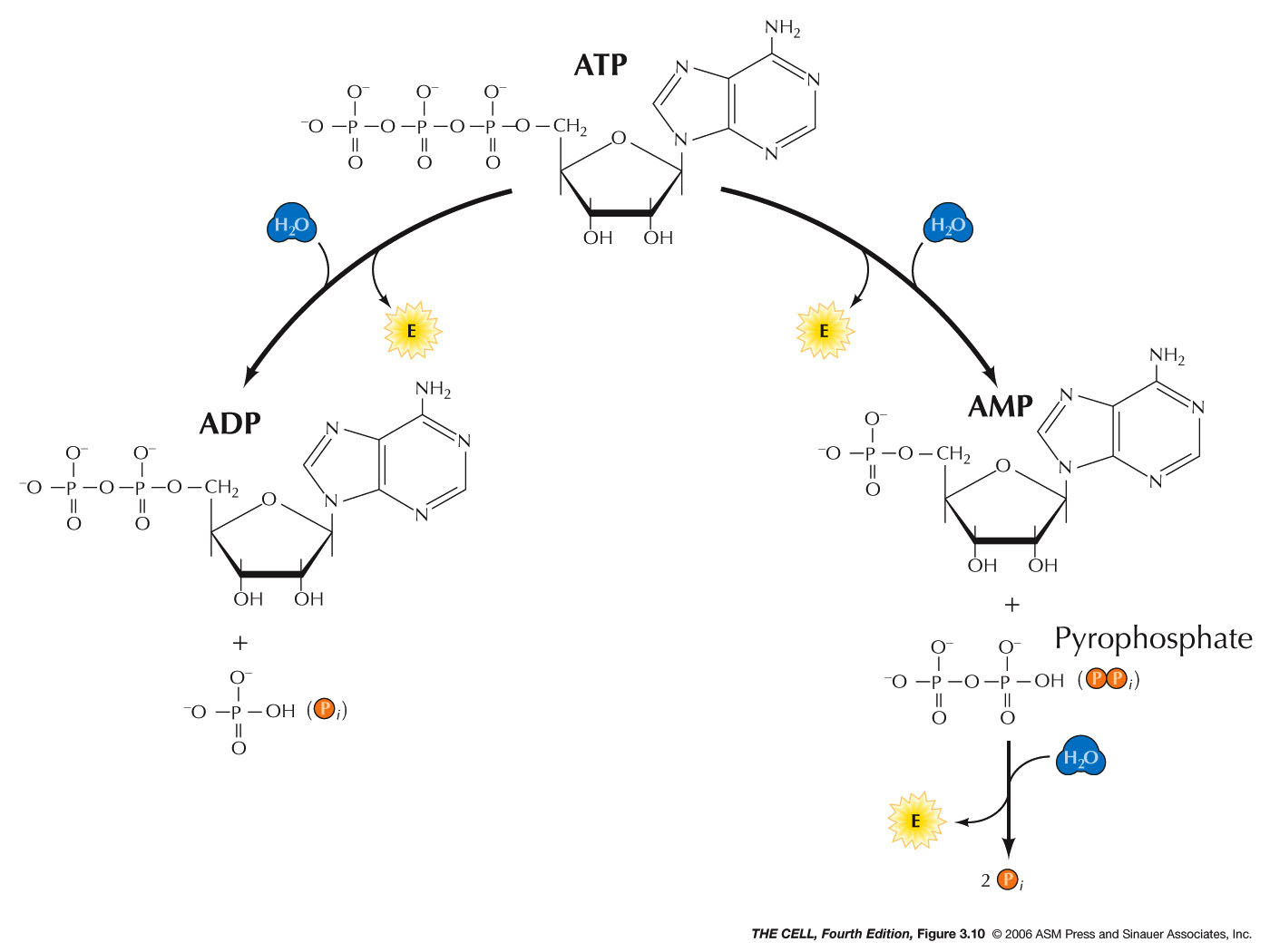
- Energy and Metabolism:
In cells, energy-rich molecules, like carbohydrates and
lipids, are catabolized releasing energy which is
captured in a usable form in the nucleotide called
adenosine 5'-triphosphate (ATP).
Various catabolic reactions are coupled with the
synthesis of ATP from ADP + PO4--.
ATP is the energy currency of the cell and can be used
for various cellular activities. Energy is released when
ATP is hydrolyzed to ADP + PO4--.
Other catabolic reaction are coupled to the production
of NADH from NAD+
or of FADH2
from FAD. Also, guanosine 5'-triphosphate (GTP) may
substitute for ATP. (Energy in the form of GTP can be
considered equivalent to energy in ATP: GTP can react
with ADP to yield GDP and ATP).
|
 |
|
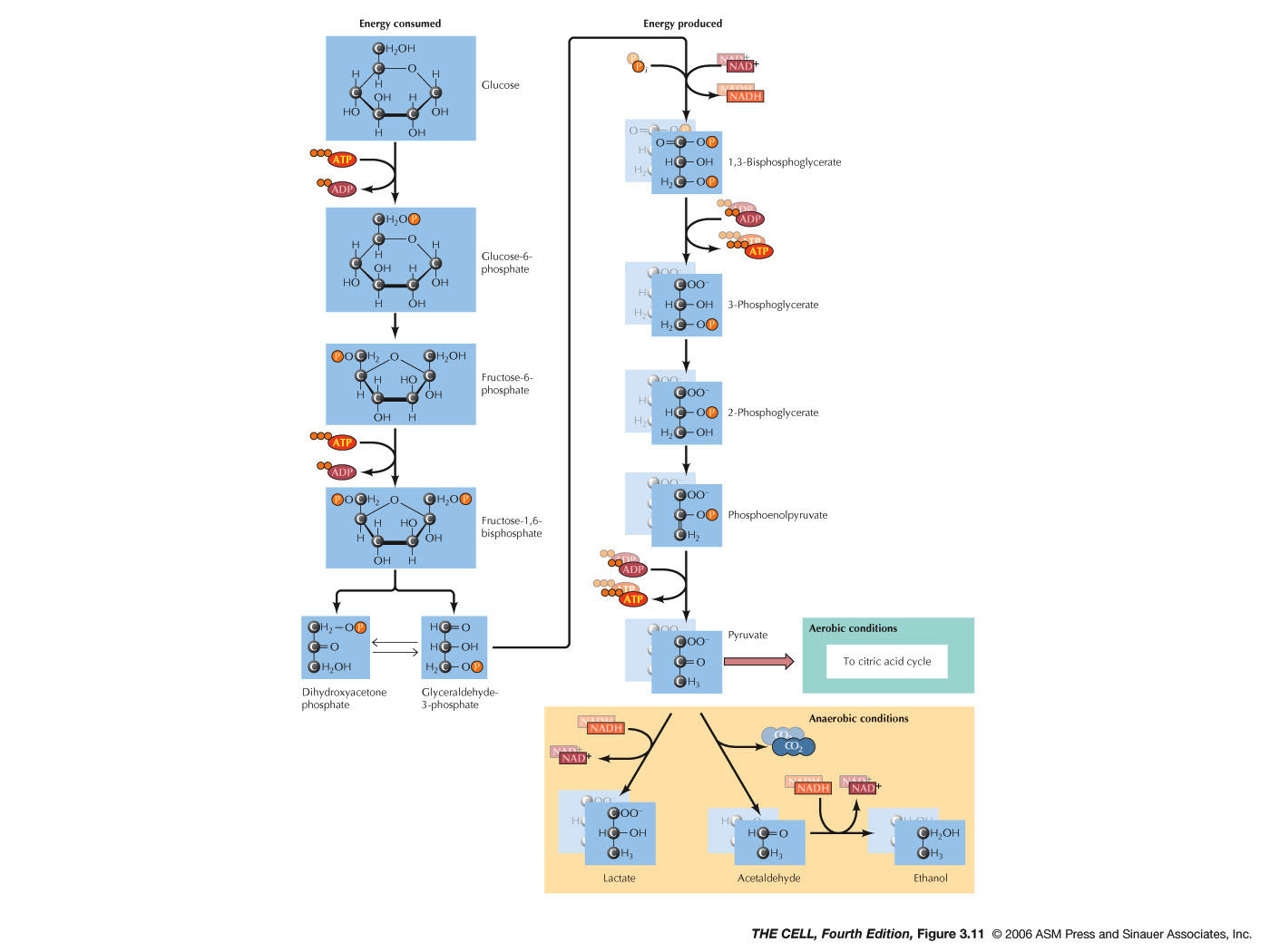
- Glycolysis: This
initial breakdown of a glucose molecule is anaerobic
and occurs in the cytosol. It converts the 6 carbon
glucose molecule into two 3 carbon molecules
(pyruvate). It requires the hydrolysis of two ATPs
during the initial steps (activation), but yields two
ATPs per pyruvate, so there is a net gain of 2 ATPs
directly from glycolysis. Also, during glycolysis one
NADH is made per pyruvate, yielding 2 NADHs. (Video)
|
 |
|
- Acetyl CoA Production
and the Citric Acid Cycle
(Kreb's Cycle, Tricarboxylic Cycle): Pyruvate
crosses the mitochondrial membrane and the rest of the
breakdown occurs within the mitochondrion. This
process is aerobic.
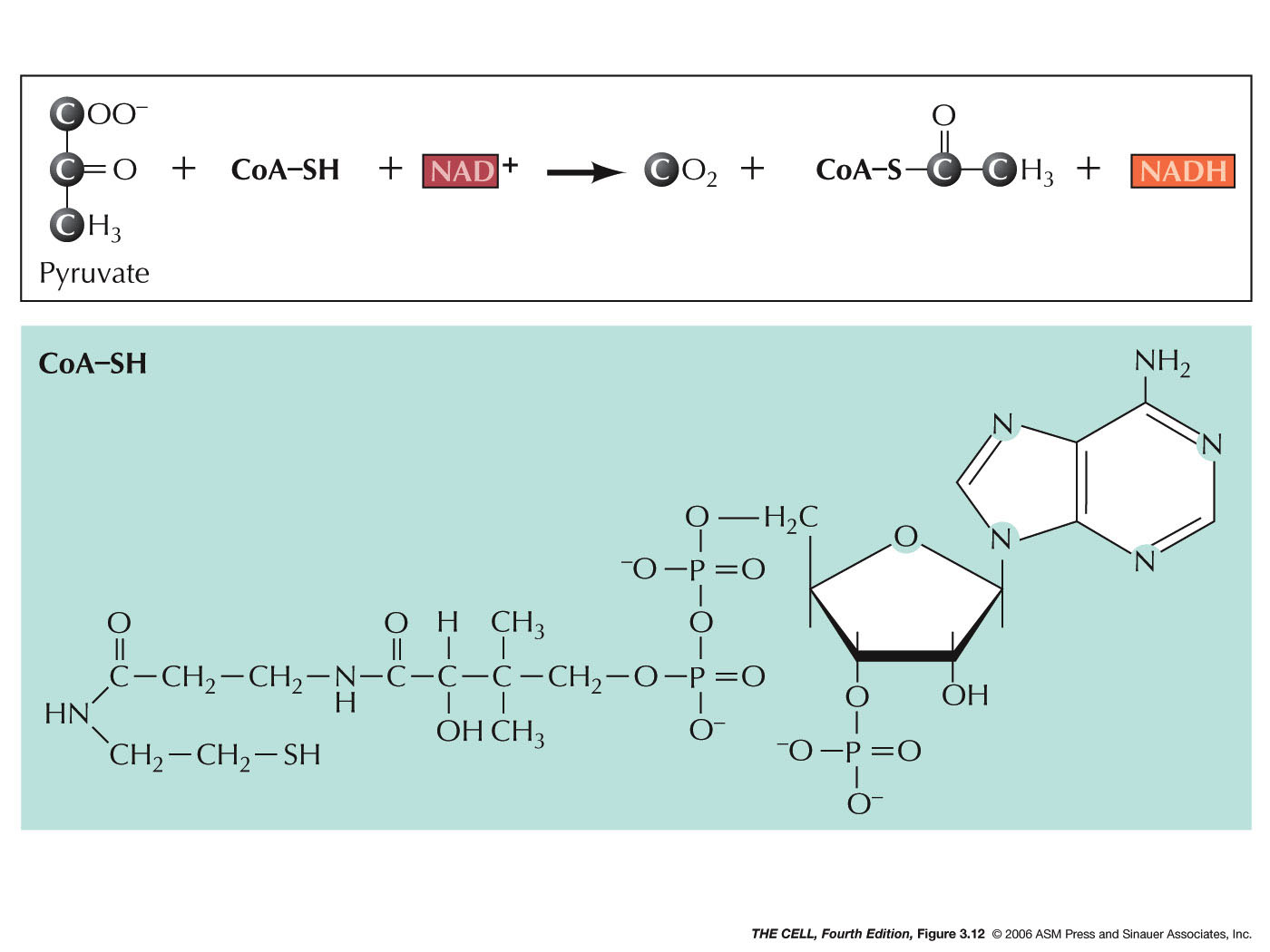
- Acetyl CoA: As
the 3 carbon pyruvate is entering the mitochondrion,
it is converted to a 2 carbon acetate with the
addition of coenzyme A (CoA) and the release of a
carbon in the form of a CO2
molecule. In the process, energy again is captured
with the reduction of one NAD+
to NADH per pyruvate. (Pyruvate + CoA + NAD+
---> Acetyl-CoA + CO2 +
NADH)
-
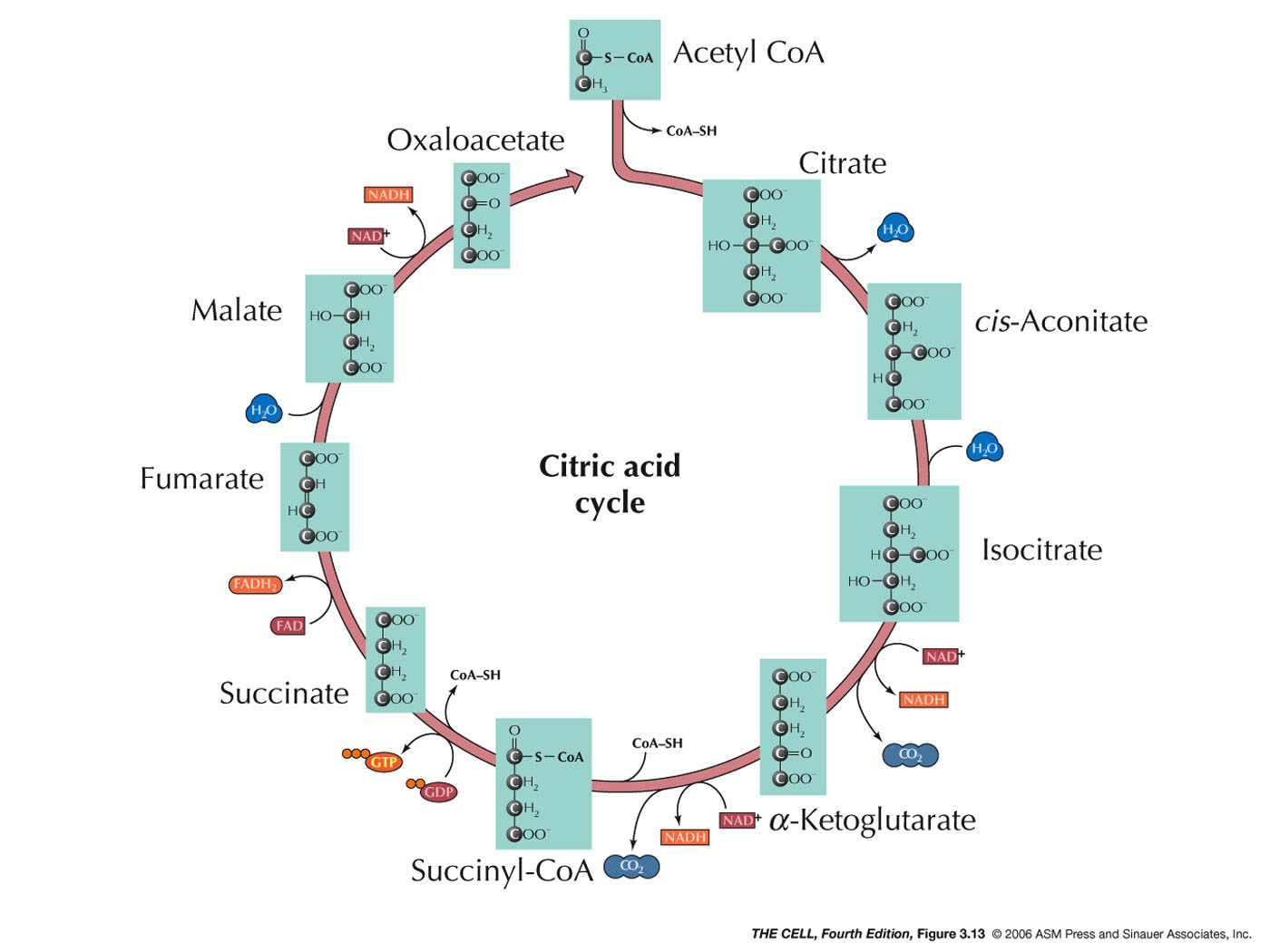
Citric Acid Cycle:
In the matrix of the mitochondrion, the complete
break down the acetate to CO 2
occurs through a series of reaction. At various
steps, energy is captured with the net gain of 1
ATP, 3 NADHs, and 1 FADH 2
per acetyl CoA. ( Video)
|
 |
|
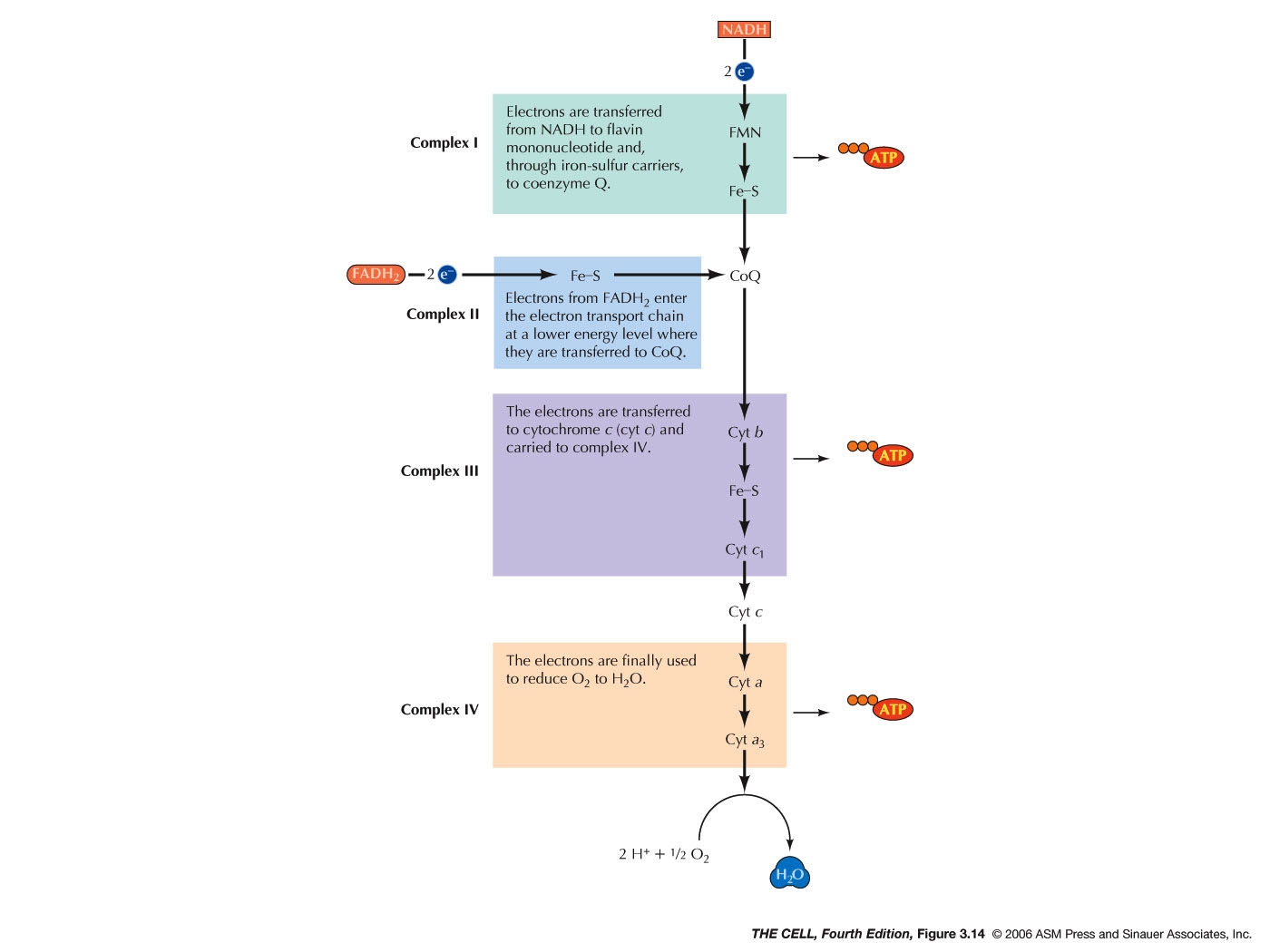
- Electron Transport
Chain:
The energy captured in NADHs or FADH2 is
converted
into ATP energy trough the electron transport system
which occurs on the inner mitochondrial membrane. This
is oxidative phosphorylation and involves the
oxidation of NADH and FADH2.
In this chain of reactions, oxygen serves at the final
electron acceptor and each NADH yields 3 ATPs while
each FADH2 yields
two.
(Video)
|
 |
|
- The breakdown of a
glucose is just one example of an energy-producing
catabolic set or reaction. All of these reactions
(anabolic and catabolic) are interconnected*
in cell metabolism.
|
 |
 405 Home
405 Home












