Samford University -- Department of Biological and Environmental Sciences
Genetics -- Biol 333 DNA
|
Nucleic
acids "will perhaps deserve equal
consideration with the proteins."
--
F. Miescher, 1871
|
|
|
The
Discovery of Nucleic Acids: In 1868,
Friedrich Miescher isolated a new substance from
nuclei of cells from hospital bandages. He realized
that this material, which he called nuclein, was a
new class of organic material. It contained C, H,
and O (like all organics), plus N (like the
proteins). However, he knew it was not protein since
it had no S and also had P. Furthermore, pepsin,
which digests proteins, had no effect on nuclein.
The nuclein Miescher isolated was probably a mixture
of DNA and RNA. Today, instead of nuclein, we call
this class of organic chemical the nucleic acids.
|
|
|
DNA
Is the Genetic Material (of almost everything):
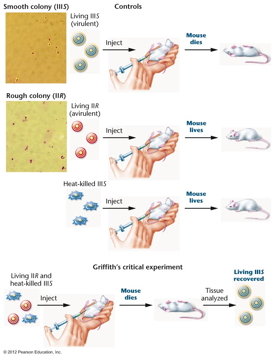
In 1944, Avery, MacLeod, and McCarty showed that DNA
was the genetic material of a bacterium (Streptococcus
pneumoniae) that causes a type of pneumonia
in mice. This experiment was based on the 1928
experiment of Griffith where he showed that some
substance from dead IIIS bacteria (S cells have a
capsule) could transform live IIR bacteria (R cells
lack a capsule) into live IIIS cells. Avery et al. showed
that Griffith's "transforming principle" was IIIS
DNA. (A 1952 experiment by Hershey and Chase
with the phage T2 showed that this virus' genetic
material was DNA. Some DNA viruses have
single-stranded DNA and some have double-stranded
DNA.) Mutagenesis experiments also indicated that
DNA was the genetic material of eukaryotes (this was
later proved directly).
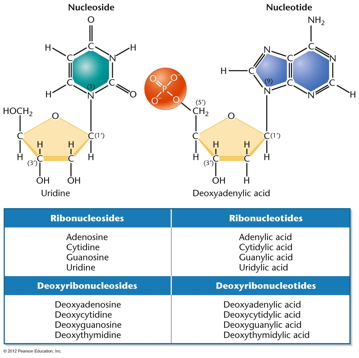
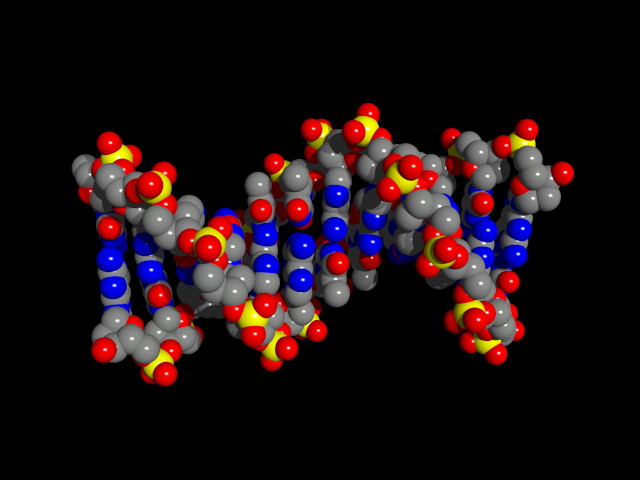
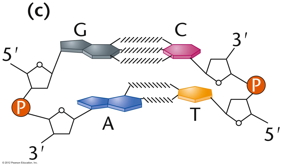
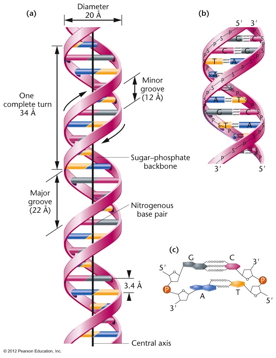
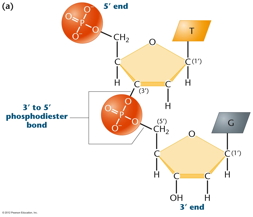
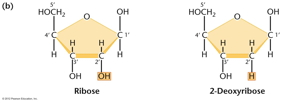
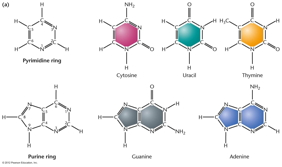
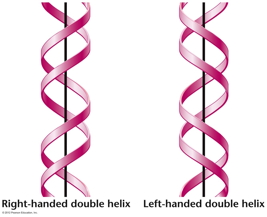
RNA Is the Genetic Material of Some Viruses. Some viruses have no DNA in the virus particle, but do have RNA. In these viruses, the RNA (which may be single stranded or double stranded) is the genetic material. Some RNA viruses replicate their RNA (similarly to the way DNA is replicated but using RNA replicase) while others use the enzyme reverse transcriptase to make a DNA intermediate which is then copied into new RNA. These viruses are called retroviruses. DNA Structure: DNA is a polynucleotide (polymer of nucleotides). (Arsenic-based life finding fails follow-up) |
|
|
|
 |
|
|
 |
|
 |
|
|
|
|
|
|
|
 |
|
|
| Things
I Learned at the Movies: The Eiffel Tower can be seen from any window in Paris. |
|
 Lectures
Lectures Online Lectures, Quizzes, and Tutorials
Online Lectures, Quizzes, and Tutorials